Balixafortide (POL6326)
A potent blocker of CXCR4, a key molecule involved in tumor growth and metastasis
Breast cancer therapy
Breast cancer is the most common cancer in women, having caused an estimated 627,000 deaths in 2018 (Global Health Estimates, WHO 2018). Despite advances in targeted hormonal and cytotoxic therapies, the overall survival in advanced metastatic breast cancer remains poor.
Many cancer drugs are usually more effective when given in combination. The rationale for combining different anticancer agents is to use drugs that work via different mechanisms, thereby increasing the likelihood of higher response rates, superior safety and efficacy and less resistance development compared to monotherapy1.
While improving patient outcome, new combination partners for established therapies ideally shall not further limit the tolerability and have low or no cytotoxic effects on its own. Balixafortide has shown very promising efficacy in its Phase 1b study in combination with eribulin (Halaven®) in patients with metastatic breast cancer2. Balixafortide is a potent and selective CXCR4 antagonist optimized for the lowest possible cytotoxicity which makes balixafortide an ideal combination partner for chemotherapy in cancer3.
A potent, selective CXCR4 antagonist
Candidate combination product for breast cancer and other cancer indications
Balixafortide is a potent and highly selective blocker of CXCR4. CXCR4 belongs to the transmembrane receptor class of G-coupled protein receptors (GPCR). CXCR4 is expressed in a variety of cancer types, and additionally, in the local tumor micro-environment by cells of the immune system 4, 5,6 . CXCR4 plays an important role in the metastatic process and allows tumor cells to migrate to sites where CXCL12, the natural ligand of CXCR4, is expressed, for example, into the bone marrow of breast cancer patients. We and others have demonstrated in preclinical cancer models a profound anti-tumor, anti-metastatic and pro-survival effect by latest generation of CXCR4 antagonists7.
Balixafortide’s key attributes2,8:
- Potent and highly selective CXCR4 inhibitor with anti-cancer response on multiple levels
- Not cytotoxic at clinically relevant doses, ideal for drug combinations
- Resulted from a stringent selection process to obtain favorable physicochemical properties without no CYP or HERG inhibition up to maximum tested concentrations
- Potentially best-in-class relative drug exposure, compared to other CXCR4 antagonists in development.
- Clear dose-response in the Phase 1b study in metastatic breast cancer, across all the efficacy endpoints.
References
1. Bayat Mokhtari et al. Oncotarget 2017; 8:38022-38043
2. Pernas et al. Lancet Oncol. 2018 Jun;19(6):812-824)
3. Sledge GW. Proc Natl Acad Sci U S A. 2019 Mar 12;116(11):4769-4771
4. Burger JA, Kipps TJ. Blood. 2006;107(5):1761-1767
5. Liotta LA. Nature. 2001;410(6824):24-25
6. Balkwill F. Semin Cancer Biol. 2004;14(3):171-179
7. Xiang et al. Mol Cancer Ther. 2015 Nov;14(11):2473-85. doi: 10.1158/1535-7163
8. Zimmermann et al. Annals of Oncology (2018) 29 (suppl_8): viii90-viii121. 10.1093/annonc/mdy272
Preclinical summary
CXCR4 also plays a critical role in tumor growth, survival, angiogenesis and metastasis9. High CXCR4 levels have been detected in almost all human tumor types, including breast cancer. High CXCR4 expression is known to correlate with aggressive metastatic behavior of cancer cells and a poor prognosis10. CXCR4 is a key player of innate and acquired immunity, both actions of the natural defense system in patients against cancer.
The anti-cancer effects of our latest generation CXCR4 antagonists such as balixafortide include direct suppression of metastatic spread, sensitization of tumor cells to chemotherapy, control of blood supply to the tumor, and favorable modulation of the immune system11,12.
CXCR4 is expressed on the surface of various immune cells, suggesting balixafortide could be involved in innate and acquired immunity. Indeed, in a previous clinical trial balixafortide led to mobilization of immune cells in healthy volunteers13. Accordingly, transiently higher white blood cell, neutrophil and lymphocyte counts were seen in the majority of breast cancer patients receiving balixafortide in combination with eribulin14. Previous in vivo and in vitro data showed that CXCR4 antagonism mitigates CD4+ T-cell exhaustion15, reverts the suppressive activity of cancer cell-protecting T-regulatory cells and M2 macrophages16, activates cytotoxicity of cancer-attacking immune cells17 and modulate immunotherapy with anti-PD-1 18, emphasizing the positive immunomodulatory function of CXCR4 antagonism. Moreover, a beneficial survival effect of balixafortide in combinatorial animal studies was reported. Such longer event-free and overall survival in breast cancer patients was also observed in the Phase 1 combination trial of balixafortide combined with eribulin2.
Based on its multifaceted modes of action, it is suggested that CXCR4 antagonism could play a crucial role in a range of therapies including chemotherapy and immunotherapies19.
Clinical proof-of-concept has been achieved in a Phase Ib/proof of concept study in combination with eribulin in patients with metastatic HER2-negative breast cancer. Balixafortide in combination with eribulin produced a higher response rate than published data for eribulin alone in a similar patient population.
Designing new medicines from a natural product
CXCR4 overexpression has been detected in more than 23 different human cancer types and correlates with a poor prognosis. CXCL12 activates distinct intracellular signaling pathways in tumor cells and stimulates proliferation, migration and invasion. However, CXCR4 is also expressed in multiple normal cells, including lymphocytes, hematopoietic stem cells, and endothelial and epithelial cells, where it contributes to the regular functioning of organs and tissue. It is hypothesized that reversible CXCR4 inhibition by well-tolerated, selective, potent and CXCL12-competitive antagonists such as balixafortide could have advantages compared to long-term blockade by therapeutic antibodies which induce apoptosis and have a limited tolerability.
The starting point for the design of such novel CXCR4 antagonists was polyphemusin, a naturally occurring peptide isolated from the horseshoe crab (Limulus polyphemus).
Polyphor has applied its PEM Technology to the discovery and optimization of fully synthetic cyclopeptide CXCR4 antagonists. This has led to balixafortide, now in Phase III clinical development, amongst other candidate CXCR4 antagonists.
Structural models indicate that balixafortide occupies a large part of the receptor pocket when complexed with human CXCR4 (see illustration).
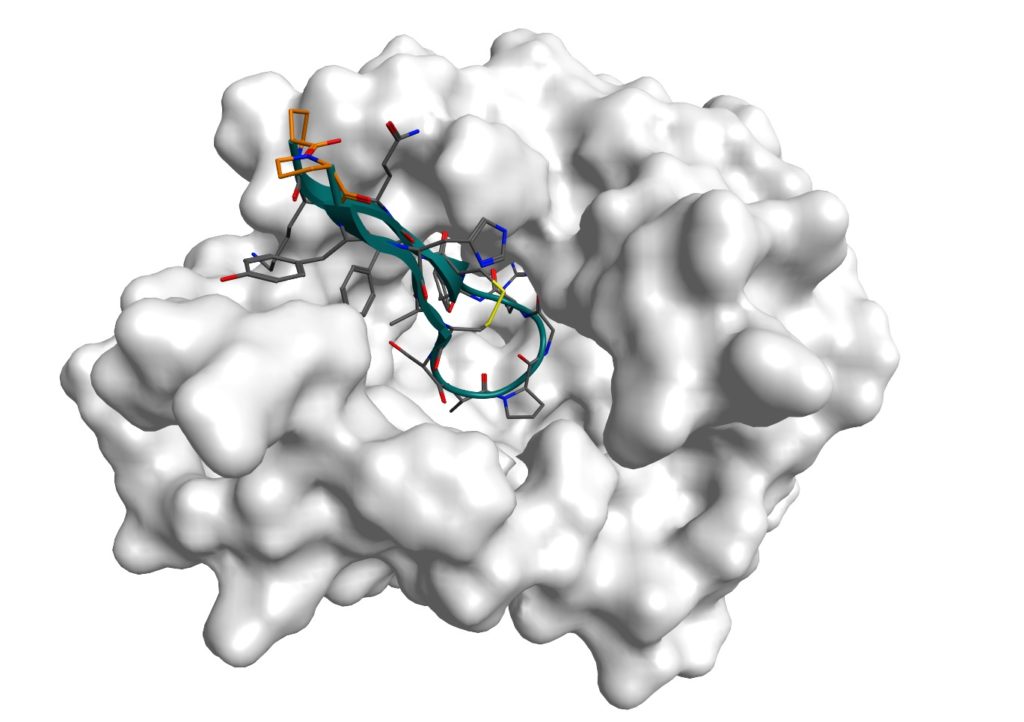
Illustration: Surface representation of the binary complex of human CXCR4 with Balixafortide*
*Data on file
References
9. Otsuka S, Bebb G. J Thorac Oncol. 2008;3(12):1379-1383.
10. Chatterjee S, Behnam Azad B, Nimmagadda S. Adv Cancer Res. 2014; 124:31-82.
11. Scala S. Clin Cancer Res. 2015;21(19):4278-85 .
12. Zimmermann et al. Mol Cancer Ther 2019;18(12 Suppl):Abstract nr A003. doi:10.1158/1535-7163.TARG-19-A003).
13. Karpova et al. J Transl Med. 2017 Jan 3;15(1):2
14. Zimmermann et al. Proceedings: AACR Annual Meeting 2019; March 29-April 3, 2019
15. Ramonell KM et al. PLoS One. 2017 Dec 12;12(12):e0188882
16. Chatterjee M et al. Cell Death Dis. 2015 Nov 19;6:e1989
17. Santagata S et al. Oncotarget 2017;8:77110–20
18. Chen Y et al. Hepatology 2015;61:1591–60
19. Cortés J, Holgado E, Perez-Garcia Oncotarget. 2018 Sep 11;9(71):33442-33443
Clinical studies – Proof of Concept
As a result of its pharmacological profile, Balixafortide has the potential to enhance the anti-tumor efficacy of chemotherapy
Results from a Phase Ib study in heavily pretreated metastatic breast cancer patients with the combination of Balixafortide and eribulin (Halaven®), an approved non-taxane inhibitor of microtubule dynamics 2,20 :
- Balixafortide shows a good safety profile, with transient and mainly mild to moderate histamine-like reactions or infusion-related reactions easily manageable with anti-histaminic agents or with a reduced infusion rate
- The addition of Balixafortide to eribulin was well tolerated without the need to reduce the dose of eribulin
- In the expanded dose cohort (n=24) the majority of patients were on treatment for 6 months or longer (up to >1 year)
- Pharmacokinetic data showed consistently reproducible dose-linear exposure with low inter-subject variability
- Balixafortide produced high tumor response rates in late stage and heavily pretreated metastatic breast cancer patients when used in combination with eribulin
- The combination of Balixafortide and eribulin resulted in a response rate of 38%. This compares favorably with published data for eribulin alone in similar patient populations (response rate 12-14%)
- In the Phase 1b trial, all other endpoints compare favorably with published data for eribulin alone in the high dose cohort (clinical benefit rate 63%, progression free survival 6.2 months, overall survival 18 months vs 28%, 3.6 months and 13.1 months for eribulin alone respectively; indirect comparison of “Embrace” Registration Trial for Eribulin)2.
References
20. Gil-Martin M et al.J Clin Oncol. 2017;35(15_suppl):2555-2555
FORTRESS Phase III Trial
FORTRESS Phase III clinical trial for balixafortide in combination with eribulin in patients with metastatic breast cancer

- In June 9th, 2019, Polyphor announced the enrollment of the first patient in its FORTRESS clinical trial, the pivotal Phase III study evaluating balixafortide (POL6326) in combination with eribulin for the treatment of patients with HER2 negative, locally recurrent or metastatic breast cancer (MBC). As of May 13th, 2020 a total of 273 patients have already been randomized. Balixafortide is the only CXCR4 antagonist in development for breast cancer and is the most advanced CXCR4 antagonist being developed in solid tumors.
- In October 2020, Polyphor announced completion of recruitment in Phase III trial of balixafortide in metastatic breast cancer . A total of 411 patients have been recruited, including 323 in the third line cohort and 88 patients in the second line cohort. Although recruitment is closed, Polyphor will allow all patients that have already registered for the study to be enrolled. This may increase the final number of patients enrolled in the study to approximately 430. As previously communicated, data on the key primary endpoint of FORTRESS, progression free survival (PFS) in the overall population, is planned for Q4 2021. An analysis of the objective response rate (ORR) in eligible patients in third and later lines of chemotherapy is planned for Q2 2021.
- FORTRESS (POL6326-009) is an international, multicenter, randomized active-controlled, open-label Phase III trial which will investigate the efficacy, safety and tolerability of intravenous balixafortide given with eribulin versus eribulin alone in the treatment of HER2 negative, locally recurrent or metastatic breast cancer. The study will comprise a total of 384 patients with HER2 negative MBC, of which 320 patients receiving Balixafortide third or subsequent line and 64 patients receiving Balixafortide second line chemotherapy. Subject to the data Polyphor will have the possibility to submit a filing for accelerated approval approximately six months after the recruitment is completed on the basis of the analysis of the objective response rate (ORR), confirmed by an independent blinded review, and of the associated durability of response. The full approval would be based on the magnitude of Progression Free Survival (PFS) on blinded independent review, supported by an overall survival trend favoring balixafortide arm and a favorable risk-benefit profile. Balixafortide has been granted fast-track status by the FDA in 2018.
For more information about the POL6326-009 clinical trial of balixafortide, please visit clinicaltrials.gov (Identifier: NCT03786094)
The CXCR4 receptor is a very promising target in oncology where CXCR4 tumor expression correlates with poor outcomes in breast cancer and in many other solid and hematologic cancers. Polyphor plans additional studies to enhance its characterization in the CXCR4 driven immune pathway and to better understand the potential for further combinations in breast cancer as well as other combinations in other tumors.
